Bildnachweis: Juan Gärtner – stock.adobe.com.
Organoids or organs-on-chips (OoCs) are two types of three-dimensional tissue culture systems amenable for the study of complex human physiological functions. Regulators increasingly recognize the value of organoids and OoCs as valid, animal-sparing test systems and provide the regulatory foundation for their application in the drug developing process. Is this the trigger for a highly innovative, rapidly growing and lucrative biotech/pharma business? By Siro Gottardi, Ilya Chuev and Dr Thomas Meier
Organoids are cellular structures derived from the self-assembly of stem cells under specific culture conditions or from tissue explants, closely resembling and thus mimicking entire human organs. OoCs are microfluidic-based devices housing cells or organoids and allowing experimentation under controlled conditions. The application of organoids and OoCs systems are well positioned to support and accelerate the drug development process.
The most recent announcement by the US FDA in relation to their regulatory position on organoid-based drug discovery was published on 10th April this year as an 11-page “Roadmap to Reducing Animal Testing in Preclinical Safety Studies” (1).
The plan aims to “improve drug safety” by using alternative ways to conduct toxicology studies in the preclinical drug development phase, specifically encouraging the use of organoids and micro-physiological systems, otherwise known as “organs-on-chips” (OoCs). Whilst organoid/OoC systems may not be ready immediately to fully replace animal testing at the present time, there is a clear path positioning these advanced cell culture systems as a key element for healthcare research.
Current and future applications
Organoids and OoC models better mimic human organ complexity and disease than animal models, avoiding inter-species differences and improving human disease relevance of test results. Their use reduces animal testing, addressing ethical concerns while enhancing the accuracy and translatability of preclinical results. These systems also enable faster drug discovery through scale-up, high-throughput screening, real-time monitoring, and personalized analysis based on patient-specific factors.
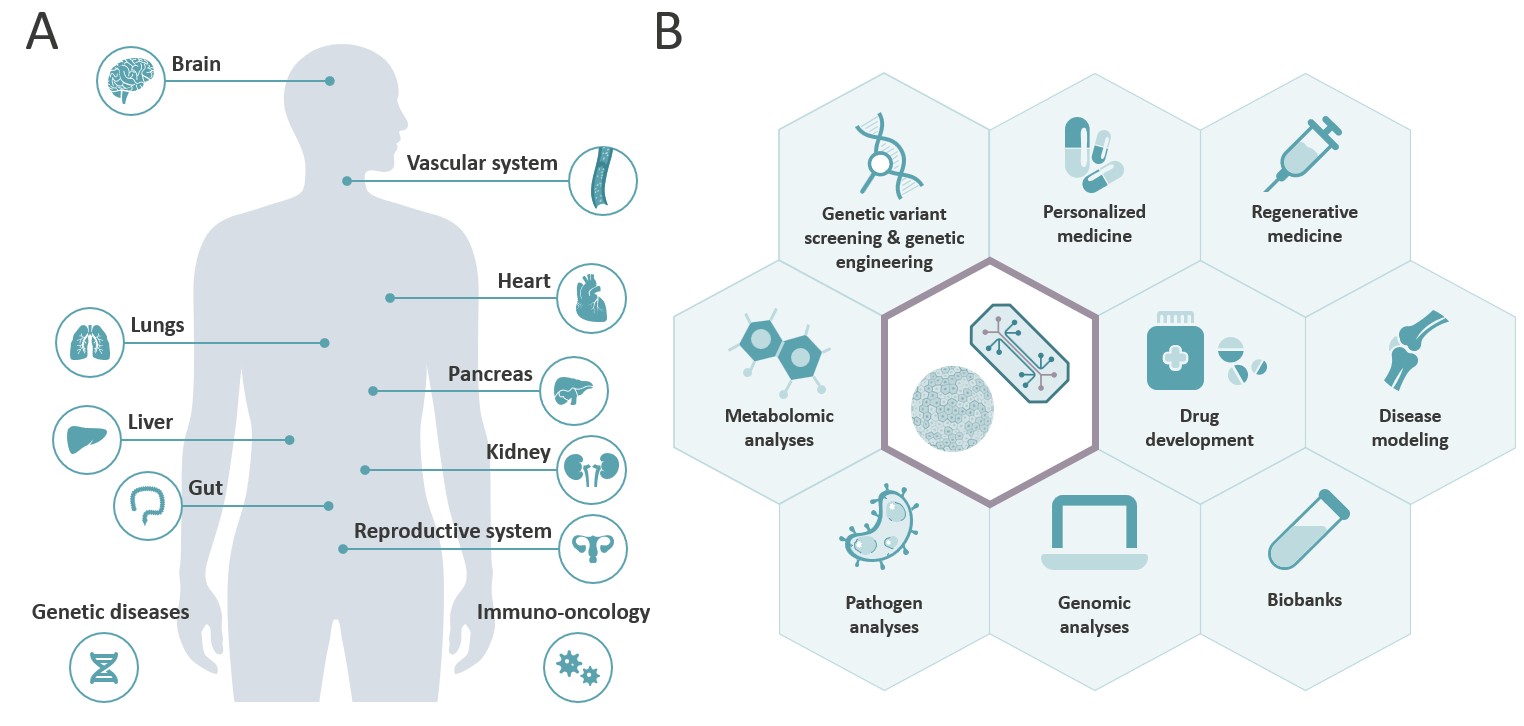
Organoid/OoC systems are already available from and mimic the functions of numerous human tissues and organs (Fig. 1A). For example, brain organoids are used to test the neurotoxicity of pesticides, liver organoids to test the hepatotoxicity of drug candidates, and multi-OoCs to study the pharmacokinetics and toxicokinetic effects of drugs (2). Use cases for organoids/OoCs range from drug discovery and profiling to personalized and regenerative medicine to disease modelling and biobanking (see Fig. 1B).
Tissue sources and use cases for organoids and OoC-systems
More recently, patient-derived organoids (PDOs) have been developed whereby organoids are derived from patients’ adult stem cells. PDOs can be implemented from nearly all epithelial organs, allowing the establishment of new assay formats with specific phenotypic readouts and relevance for various diseases (3). For example, PDOs may become important tools in cancer research, as cell monolayers derived from a patient tumor reflect intercellular interactions, deregulated cell cycle and enhanced proliferation rates compared to in vivo tissue cells; and these individual PDOs can help to mimic and explain variations of drug responses.
Favorable Regulatory Environment
Culminating in the recent “Roadmap” publication, the FDA’s evolving view on the potential of organoids/OoCs in the drug development process started in December 2022 with the FDA Modernization Act 2.0 and is part of a broader initiative to incorporate so called New Approach Methodologies that reduce reliance on traditional animal testing. As the FDA recognizes that “organoids can enhance the predictive accuracy of preclinical studies, potentially reducing the high attrition rates observed in clinical trials due to unforeseen toxicities or lack of efficacy”, the Agency has begun to accept data from organoid-based studies as part of Investigational New Drug (IND) applications, provided that sponsors can provide evidence that models used are validated and reliable in predicting whole-body responses.
In response to the emerging use of organoids, the EMA has established the Non-Clinical and New Approach Methodologies European Specialized Expert Community (4) to “facilitate the integration of innovative in vitro models, including organoids, into the drug development process”. Furthermore, the EMA offers a voluntary Qualification of Novel Methodologies procedure, allowing developers to seek scientific advice or qualification opinions on innovative methods like organoids.
The Chinese government also has recognized the potential of organoid and OoC technologies and has included them in its national strategic plans, such as the Healthy China 2030 Plan. In 2024, the Center for Drug Evaluation of China’s NMPA issued a document entitled ‘‘Technical Guidance Principles for Non-Clinical Research of Human Stem Cell Products,’’ which mentioned the use of alternative methods, such as organoids and OoCs, to obtain supplementary information on product efficacy and safety (2).
Whilst these national/supra-national initiatives are forthcoming and paving the way to a more widespread use of organoids/OoCs, there are currently no ICH guidelines specifically defining use criteria during drug development. However, the ICH mentioned the use of organoids and other alternative methods in the documents of ICH E14 and ICH S12 (2).
Remaining regulatory challenges
Current challenges that need to be addressed to allow a broader regulatory acceptance of organoids/OoCs include (i) standardization and validation by ensuring reproducibility and robustness across different laboratories, (ii) scalability by establishing scalable production methods that comply with current pharmaceutical standards and (iii) regulatory qualification by demonstrating the reliability and relevance of organoid models in predicting human responses (5).
The growing global organoid/OoCs market
The global market for organoids and OoCs was estimated to be between $800 million and $2.8 billion in 2024 with an anticipated CAGR of 11-23% in coming years (6-10). One report (9) stated that organoids derived from the intestine were taking the lead in 2023 with a market share of 32%. In terms of application, developmental biology accounted for a significant share of 41.3%, while academic and research institutes held the largest revenue share at 47.5% in the organoids market.
Currently, services offered by companies could be categorized into (i) laboratory infrastructure and equipment, (ii) (one-time) consumables, (iii) software solutions for experiment control and data analysis and (iv) classical CRO-type business models. Drivers for future growth come from the increasing number of organ transplantation procedures and the rising number of experiments in genetic disease. The remaining challenges for a more wide-spread use still result from inadequate infrastructure for 3D cell cultures and difficulty in incorporating human organs in workflows (10).
We analyzed a total of 30 companies with a business model centered around organoids/OoCs, of which 14 (46%) are domiciled in Europe (of which 4 (13%) in the DACH region), 13 (43%) in the US and Canada, 2 (6%) in China and 1 (3%) in Israel. Whilst currently the majority (80%) of these companies have their core business in improved tissue culture systems and devices, we also see an emerging trend of implementation of AI-based analysis systems, allowing better ways to characterize test items in the context of drug candidate profiling.
Clearly, the favorable position of US, European and Chinese regulators towards the use of organoids/OoC systems and the increasing desire to accelerate and de-risk the drug development process and at the same time spare animal use should be an excellent basis for an attractive, rapidly growing biotech/pharma business case.
References:
1) FDA: Roadmap to Reducing Animal Testing in Preclinical Safety Studies. https://www.fda.gov/media/186092/download and citations therein
2) Zhuo et al., (2025). Organoids and organs-on-chips: Recent advances, applications in drug development, and regulatory challenges. Med 6, 100667, April 11, 2025 https://doi.org/10.1016/j.medj.2025.100667 and citations therein
3) Bünin & Reckzeh (2025). Opportunities of patient-derived organoids in drug
development. British Journal of Pharmacology, 1–6. https://doi.org/10.1111/bph.70010
4) EMA: https://www.ema.europa.eu/en/committees/working-parties-other-groups/chmp/non-clinical-new-approach-methodologies-european-specialised-expert-community?utm_source=chatgpt.com
5) Vives & Batlle-Morera (2020). The challenge of developing human 3D organoids into medicines. Stem Cell Research & Therapy 11:72; https://doi.org/10.1186/s13287-020-1586-1
6) Organoids Market Size, Growth, Trends 2035 | MRFR
7) https://www.futuremarketinsights.com/reports/organoids-market
8) Human Organoids Market Size, Share | Industry Report, 2030
9) Market.us
10) researchnester.com